In the modern pharmaceutical landscape, impurities are no longer viewed as secondary concerns. They are central to drug safety, regulatory compliance, and analytical reliability. Among antifungal therapies, Terbinafine remains one of the most widely prescribed agents for dermatophytic infections, making its impurity profile a key focus area for API manufacturers, analytical laboratories, and regulatory teams alike.
For January 2026, Advent Chembio’s Molecule of the Month spotlights N-Desmethyl Terbinafine, a scientifically significant and regulatorily relevant impurity that plays a vital role across impurity profiling, method development, stability studies, and ICH-compliant impurity control strategies.
Terbinafine and the Importance of Impurity Characterization
Terbinafine is an allylamine antifungal drug that exerts its effect by inhibiting squalene epoxidase, a key enzyme in fungal sterol biosynthesis. Its proven efficacy and broad clinical use have resulted in large-scale API manufacturing, where consistent quality and impurity control are paramount.
During synthesis, storage, and metabolism, Terbinafine can give rise to structurally related impurities. These impurities may originate from:
Process-related side reactions
Incomplete methylation steps
Degradation under stress conditions
In vivo metabolic pathways
Among these, N-Desmethyl Terbinafine stands out due to its close structural resemblance to the parent API and its frequent occurrence in analytical and stability studies.
What Is N-Desmethyl Terbinafine?
N-Desmethyl Terbinafine is a process-related impurity and known metabolite of Terbinafine, formed through the demethylation of the nitrogen atom in the parent molecule. This minor structural change retains much of the molecular backbone, making the impurity analytically challenging to separate and quantify.
Because of this similarity, N-Desmethyl Terbinafine is routinely used as a reference impurity to demonstrate method selectivity and robustness—two aspects that regulators closely scrutinize.
Scientific and Regulatory Significance
1. Essential Reference Impurity for Impurity Profiling
In impurity profiling studies, pharmaceutical manufacturers must demonstrate that known impurities can be reliably detected and quantified. N-Desmethyl Terbinafine is commonly included as a reference standard during:
HPLC and UPLC method development
Method validation and verification
System suitability testing
Impurity limit justification
Its chromatographic behavior—often eluting close to Terbinafine—makes it an ideal marker to challenge analytical methods and ensure accurate separation.
2. Role in Stability and Forced Degradation Studies
Stability testing is a cornerstone of API development and lifecycle management. During forced degradation and long-term stability studies, N-Desmethyl Terbinafine may emerge as a degradation-related impurity under specific stress conditions.
Its presence helps scientists:
Identify degradation pathways
Track impurity trends over time
Confirm method stability-indicating capability
A well-characterized reference standard ensures that observed impurity peaks are correctly identified and quantified.
3. Supporting ICH-Compliant Impurity Control Strategies
ICH guidelines such as ICH Q3A (Impurities in New Drug Substances) and ICH Q3B (Impurities in New Drug Products) require comprehensive control of impurities above defined thresholds.
N-Desmethyl Terbinafine is particularly relevant because:
It is a known and structurally related impurity
It may require qualification depending on exposure levels
It often appears in regulatory impurity discussions
Availability of a qualified impurity standard simplifies regulatory submissions and strengthens data credibility.
4. Toxicological and Pharmacokinetic Relevance
Beyond analytical chemistry, N-Desmethyl Terbinafine also holds value in toxicological and pharmacokinetic evaluations. As a metabolite of Terbinafine, it may be observed during metabolism studies and safety assessments.
Understanding its molecular identity, concentration, and behavior supports:
Risk assessment activities
Justification of impurity acceptance criteria
Regulatory communication and scientific rationale
Product Overview: N-Desmethyl Terbinafine at Advent Chembio
As part of its commitment to supplying high-quality pharmaceutical impurities and API working standards, Advent Chembio has successfully developed and characterized N-Desmethyl Terbinafine with a focus on analytical reliability and regulatory usability.
Key Product Details
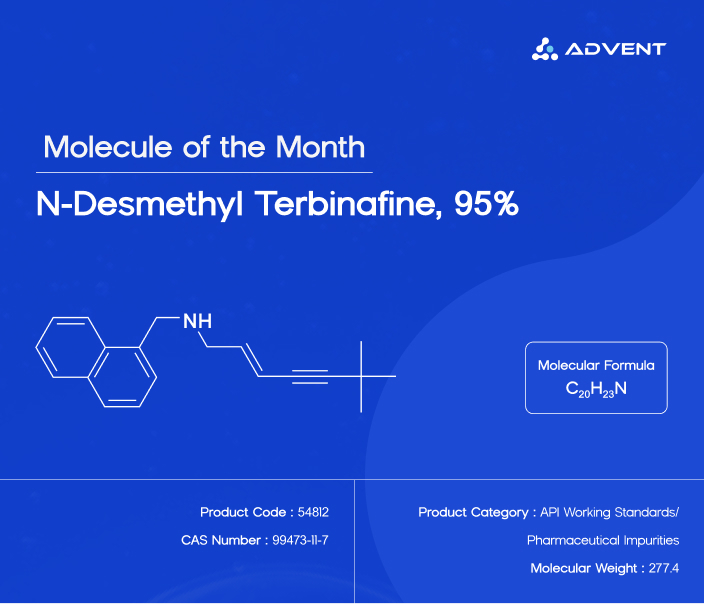
Product Name: N-Desmethyl Terbinafine, 95%
Product Category: API Working Standards / Pharmaceutical Impurities
Chemical Name:
N-[(2E)-6,6-Dimethyl-2-hepten-4-ynyl]-1-naphthalenemethanamineSynonym: Demethylterbinafine
Product Code: 54812
CAS Number: 99473-11-7
Molecular Weight: 277.4
Molecular Formula: C₂₀H₂₃N
Comprehensive Analytical Support
To ensure suitability for regulated applications, Advent Chembio provides robust analytical characterization for N-Desmethyl Terbinafine, including:
Purity determination by HPLC
¹H NMR spectroscopy
Infrared (IR) spectroscopy
Mass spectrometry
Each batch is supplied with a Certificate of Analysis (CoA), and potency data is provided wherever applicable—enabling confident use across R&D, QC, and regulatory workflows.
Ready Stock and Prompt Delivery
One of the major challenges faced by analytical and development teams is the lead time associated with sourcing qualified impurity standards. Custom synthesis often introduces delays, impacting project timelines.
N-Desmethyl Terbinafine from Advent Chembio is available in ready stock, allowing for prompt delivery and uninterrupted progress across development, validation, and stability programs.
Why High-Quality Impurity Standards Matter
Impurity standards are only as valuable as their characterization. Poorly defined or inconsistent standards can compromise:
Method validation outcomes
Stability data interpretation
Regulatory confidence
By offering a well-characterized, high-purity N-Desmethyl Terbinafine standard, Advent Chembio helps laboratories maintain data integrity, compliance, and analytical excellence.
Molecule of the Month: Driving Knowledge and Quality
The Molecule of the Month initiative goes beyond product highlighting. It is designed to:
Showcase scientifically relevant molecules
Educate the industry on impurity significance
Support best practices in analytical and regulatory science
Each featured molecule is selected based on its practical importance in real-world pharmaceutical development.
Conclusion
N-Desmethyl Terbinafine exemplifies the critical role impurities play in ensuring the quality and safety of widely used APIs such as Terbinafine. As a process-related impurity and metabolite, it is indispensable for impurity profiling, stability studies, analytical method validation, and ICH-compliant regulatory strategies.
With ≥95% purity, comprehensive analytical data, ready-stock availability, and strong documentation, Advent Chembio’s N-Desmethyl Terbinafine empowers pharmaceutical companies, CROs, and analytical laboratories to move forward with confidence.
It is this blend of scientific relevance, regulatory value, and practical usability that makes N-Desmethyl Terbinafine a fitting Molecule of the Month for January 2026.