Pharmaceutical research is a complex sphere, where some molecules work behind the scenes but are utterly necessary to make a breakthrough discovery. One such molecule is Fmoc-Arg(Pbf)-Arg(Pbf)-OH- a di-arginine peptide that is doubly faraday-protected and this has become the workhorse of the modern peptide synthesis processes. Although its name may appear as an alphabet soup to people who do not understand chemistry, knowing this substance gives one a reason why it is important to consider precision chemistry in drug development.
What Is So Special about this Molecule?
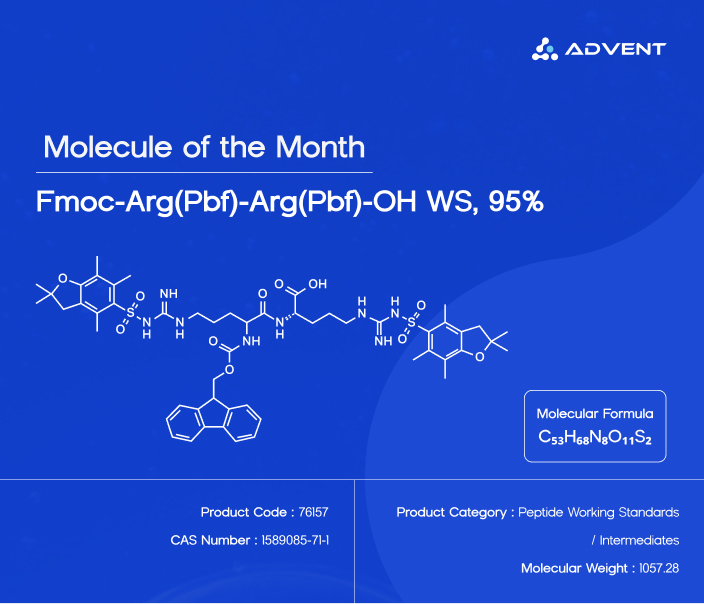
Fmoc-Arg(Pbf)-Arg(Pbf)-OH (CAS No. 1589085-71-1), is in essence the success story of molecular engineering. Having a molecular formula C53H68N8O11S2 and molecular weight of 1057.28 g/mol, this peptide intermediate is fully chemically protected (two layers) in contrast to a single one. Consider it molecular armor meant to shield the reactive sites until the appropriate time in the course of synthesis.
The N-terminus is covered by the "Fmoc" group (fluorenylmethyloxycarbonyl) which acts as a gatekeeper and the Pbf (2,2,4,6,7-pentamethyldihydro benzofuran-5-sulfonyl) which covers the guanidino side chains. This two layer security system eliminates undesirable chemical interactions throughout the delicate process of forming peptide chains, one amino acid at a time. Arginine residues would go on a rampage without these protective groups, and make unwanted connections and thwart whole synthesis projects.
The Science Peptide Synthesis in Solid Phase
The production of therapeutic peptides changed forever, and it was enabled by solid-phase peptide synthesis (SPPS) and the use of amino acids such as Fmoc-Arg(Pbf)-Arg(Pbf)-OH. It is like a Lego block building process: every piece has to fit the right way and safeguarding groups can make sure the connection is only made when intended.
In the process of SPPS, the Fmoc group is cleavable under mild conditions when the conjugates are in basic conditions, revealing the amino terminus to the subsequent coupling reaction. In the meantime, Pbf groups do not deprotect during the entire process of synthesis because of their highly reactive guanidino groups. Such step-by-step deletion technique enables chemists to build complex peptide sequences with an unprecedented accuracy and efficiency.
Pharmaceutical Research uses in the real world
In addition to its synthesis function, this compound is also used as an important reference standard to control the quality of pharmaceuticals. In creating new peptide-based drugs, the researchers are required to detect and determine impurities with a high degree of accuracy. The calibration of these measurements is done against fmoc-Arg(Pbf)-Arg(Pbf)-OH.
Another important usage is analytical method development. Scientists should come up with consistent ways of fully characterizing any peptide therapeutic before it can be used in patients. These involve chromatographic analysis such as HPLC and mass spectrometry. A well-characterized standard that is 95 per cent pure with all the necessary analytical data, i.e., 1H NMR, IR spectroscopy, mass spectrometry, and Certificate of Analysis, is reproducible and reliable under varying laboratory and testing conditions.
The Dilemma: Managing Daintily
This is the point where theory comes into the picture: Fmoc-Arg(Pbf)-Arg(Pbf)-OH demands respect. The molecule is highly sensitive to moisture and light, which means that a clean standard can be turned into a ruined material given it is used without proper care. Photochemical processes induced by light may modify the protecting groups of the disulfide, whereas humidity may precipitate premature disulfide deprotection.
The seasoned researchers are fully aware of the procedure: a controlled storage environment, a speedy sample preparation process, and the minimal amount of light during analysis. These are not just suggestions, but necessity to keep chemicals intact. Poor standard implies poor data, this leads to poor development of the methods and may be assumed that the quality control is impaired. Patient safety is at stake in pharmaceutical research, thus this level of attention to detail is not an option.
The Future: The Increasing Function of Labeled Peptides
Diabetes, cancer, or any other illness, as the peptide therapeutics keep gaining momentum in modern medicine, the pressure on building blocks of high quality and reference standards increases. Such molecules as Fmoc-Arg(Pbf)-Arg(Pbf)-OH may not be headline grabbers, but they facilitate the science that is.
Peptide-based drugs are becoming the future of the pharmaceutical industry due to their exquisite specificity, reduced side effects, and in most cases, superior efficacy over small molecule analogs. The development of each new peptide therapeutic demands careful attention and the development cannot be achieved without reliable chemical tools. Amino acid analogs which have been safeguarded, offer the accuracy and manipulation that is needed to translate laboratory ideas into life-saving drugs.
The Bottom Line
Fmoc-Arg(Pbf)-Arg(Pbf)-OH is a good example of how advanced chemistry has facilitated innovation in pharmaceutical products. Its twofold defense policy, ability to maintain stability in the presence of synthesis conditions, and capability to be a reliable reference standard could not afford to be dispensed by researchers who are pushing the limits of peptide therapeutics. Although special caution and respect must be taken with such compounds when working with them, the reward is in the form of better medications, more dependable analysis techniques, and, in the end, better patient results.
In life, like chemistry, it is often what is preserved until the right time to be shown. This simple molecule precisely does so, one peptide bond at a time.