In the fast-evolving world of pharmaceutical research and quality assurance, precision is everything. Even the smallest impurity or structural variation can influence how a drug performs in clinical practice. That’s why researchers, formulation scientists, and quality analysts are constantly on the lookout for highly characterized reference materials to aid in method development, regulatory submissions, and long-term quality monitoring.
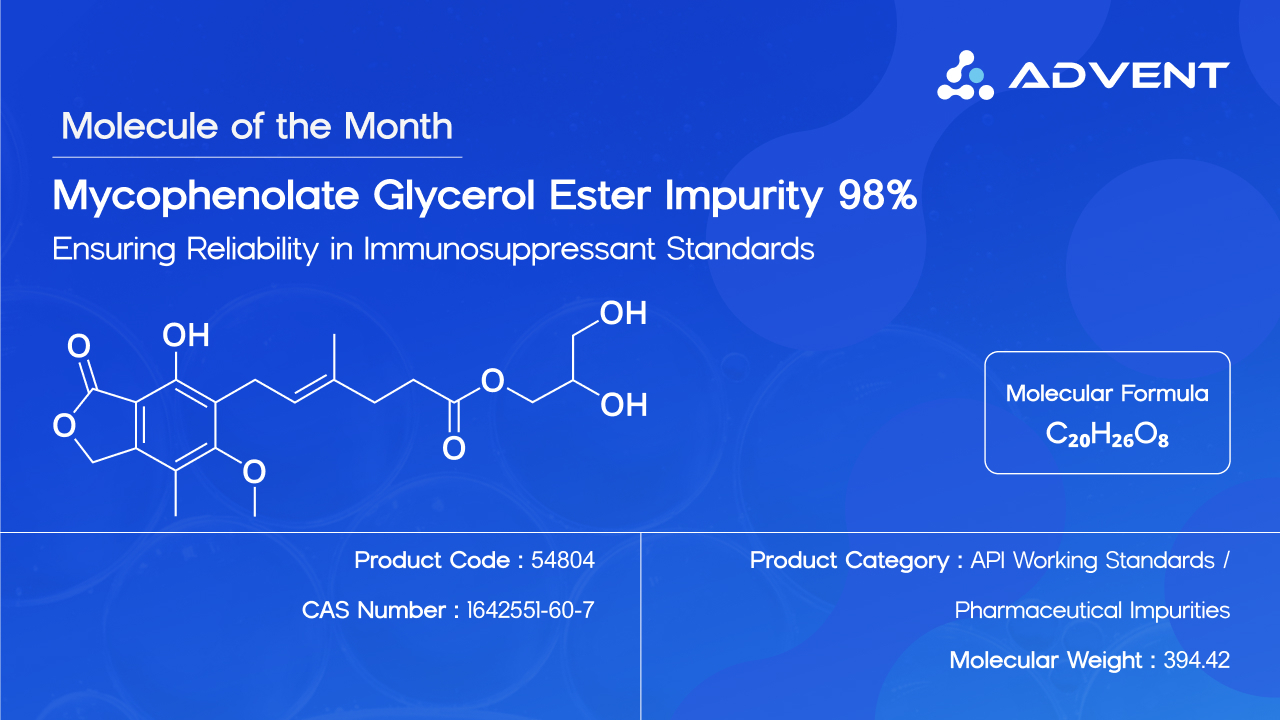
One such critical compound that has gained attention is Mycophenolate glycerol ester WS, 98%. With a purity of greater than 98%, this specialized molecule plays a pivotal role in advancing the quality framework of one of the most important immunosuppressant drugs available today—Mycophenolate Mofetil.
What is Mycophenolate Glycerol Ester?
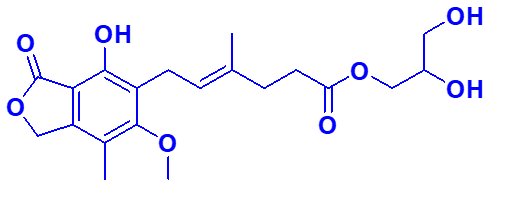
Chemically known as 2,3-Dihydroxypropyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate, Mycophenolate glycerol ester is essentially the glycerol ester derivative of Mycophenolate.
Molecular Formula: C₂₀H₂₆O₈
CAS Number: 1642551-60-7
This compound exists as a specific impurity of Mycophenolate Mofetil, a widely prescribed immunosuppressant used in organ transplantation. Detecting and analyzing such impurities is essential to ensure the safety, stability, and regulatory compliance of pharmaceutical formulations.
The Role of Impurity Standards in Pharma
In pharmaceuticals, an impurity isn’t just a trace molecule—it’s often a critical checkpoint. Regulatory agencies like the FDA and EMA require manufacturers to identify, characterize, and control impurities in active pharmaceutical ingredients (APIs) and drug products.
Here’s why Mycophenolate glycerol ester is so important:
It helps simulate real-world impurity profiles during drug stability studies.
It supports method development for separating, quantifying, and validating analytical techniques such as HPLC and LC-MS.
It ensures quality control (QC) teams can detect the presence of this impurity reliably, preventing compliance gaps.
It aids in Abbreviated New Drug Application (ANDA) filings, where regulators expect exhaustive impurity characterization.
Without access to well-defined impurity standards like Mycophenolate glycerol ester, researchers would face significant hurdles in proving their products meet international benchmarks.
Key Features of Advent’s Offering
Advent’s Mycophenolate glycerol ester is designed with the needs of researchers and manufacturers in mind. Its highlights include:
✔ >98% Purity – Ensures high analytical accuracy and reproducibility.
✔ Available from mg to g scale – Flexible for small research labs as well as industrial QC departments.
✔ Specific impurity of Mycophenolate Mofetil – Tailored for one of the most widely used immunosuppressants.
✔ Critical for ANDA, QC, and method validation – Aligns with regulatory expectations for impurity studies.
✔ Delivered with complete characterization – Supported by data such as ¹H NMR, Mass, IR, and TGA, etc, ensuring full structural and thermal analysis.
Applications Across the Drug Lifecycle
The real strength of this impurity reference standard lies in its versatility across multiple stages of the pharmaceutical pipeline:
Research & Development (R&D):
Scientists can use this compound to study degradation pathways of Mycophenolate Mofetil under stress conditions such as light, temperature, and humidity.Analytical Method Development:
Laboratories rely on impurity standards to fine-tune separation techniques and ensure consistent detection of trace impurities.Regulatory Submissions (ANDA):
Generic manufacturers need to prove their formulations are equivalent to innovator drugs. Having authentic impurity standards strengthens their submissions.Quality Control (QC):
Routine monitoring of impurities during production ensures batch-to-batch consistency, directly impacting patient safety.Validation & Audits:
With complete characterization data, this impurity standard provides confidence during third-party audits and regulatory inspections.
Scientific Characterization: A Snapshot
One of the most distinguishing aspects of Advent’s Mycophenolate glycerol ester is its robust characterization package:
¹H NMR (Nuclear Magnetic Resonance): Confirms hydrogen placement in the structure.
Mass Spectrometry (MS): Provides molecular weight accuracy.
Infrared (IR) Spectroscopy: Identifies functional groups.
Thermogravimetric Analysis (TGA): Evaluates thermal stability and decomposition profile.
This multi-layered analytical data ensures researchers are working with a trusted, verified compound, eliminating ambiguity.
Why This Matters for Mycophenolate Mofetil
Mycophenolate Mofetil is a cornerstone drug in transplantation medicine, preventing organ rejection in kidney, heart, and liver transplant patients. Given its critical role, ensuring its purity, stability, and safety isn’t optional—it’s mandatory.
Impurities like Mycophenolate glycerol ester, if not adequately controlled, could:
Compromise drug stability
Alter pharmacological activity
Trigger regulatory non-compliance
Ultimately impact patient safety
That’s why having access to high-quality impurity standards is a non-negotiable part of pharmaceutical excellence.
Advent: Driving Innovation in Pharmaceutical Impurities
At Advent, the philosophy is simple—“Innovation in Every Molecule.” By supplying a wide range of pharmaceutical impurities and working standards, the company supports scientists, formulators, and quality experts worldwide in their pursuit of reliable medicines.
Mycophenolate glycerol ester WS, 98% is just one example of how Advent bridges the gap between scientific rigor and practical application. Each molecule is not just a chemical—it’s a tool for innovation, enabling safer, more effective therapies for patients across the globe.
Conclusion: Small Molecule, Big Impact
In the pharmaceutical landscape, where every decimal point matters, impurity standards like Mycophenolate glycerol ester become silent enablers of trust, quality, and compliance. Whether you’re a researcher developing a new formulation, a QC analyst validating methods, or a regulatory professional preparing an ANDA dossier—this compound provides the confidence you need to deliver excellence.
💡 Discover Mycophenolate glycerol ester WS, 98% and more with Advent – where innovation begins at the molecular level.