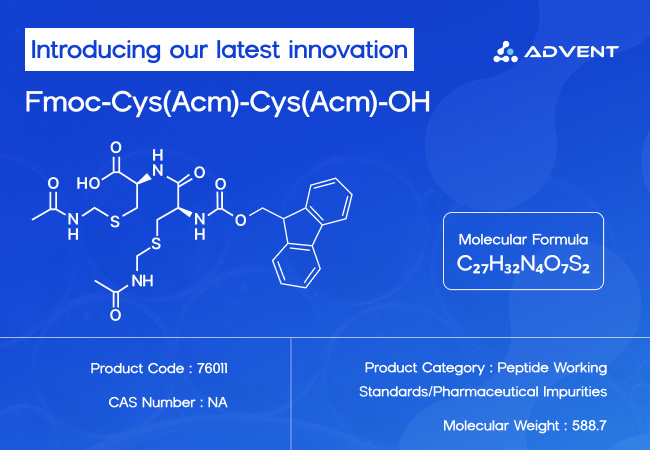
When synthesizing peptides, precision is something that cannot be compromised to any extent, and when working with cysteine residues, things can get tricky quickly. Fmoc-Cys(Acm)-Cys(Acm)-OH molecule then plays the leading role here. This protected dipeptide molecule is a go-to building block that brings control, stability and efficiency to the table. This molecule is invaluable to researchers who are developing disulfide-rich peptides.
In this blog, we discuss its structure, applications and advantages. Let’s dive in to learn more.
What is Fmoc-Cys(Acm)-Cys(Acm)-OH?
Its IUPAC name is: (2S)-2-[[(9H-Fluoren-9-ylmethoxy)carbonyl]amino]-3-[[2-[[[(2S)-2-acetamidomethyl-3-sulfanylpropanoyl]amino]acetyl]amino]propanethioic acid
It is a homodipeptide molecule having two L-cysteine residues. Each of the cysteine’s thiol group (-SH) is protected by an acetamidomethyl (Acm), preventing any wanted disulfide formation during synthesis.
The Fmoc group (fluorenylmethyloxycarbonyl) on the N-terminus protects it, making it the most suitable molecule for controlled peptide synthesis reactions.
The carboxylic acid (-OH) at the C-terminal of the molecule allows easy coupling for elongation during peptide synthesis.
Applications of Fmoc-Cys(Acm)-Cys(Acm)-OH
Here is how this dipeptide is used in the industry:
Synthesizing Disulfide-rich peptides: Peptides with multiple cysteine residues usually rely on specific disulfide bond formation to maintain their biological structure and function. Incorrect bonding in therapeutic peptides such as oxytocin, vasopressin, and conotoxins can make the compound inactive or even toxic. Since the Acm(acetamidomethyl) group is resistant to degradation in acidic and basic environments, it remains stable throughout the Solid Phase Peptide Synthesis cycles. After the chain elongation process, the Acm groups can be selectively removed using oxidizing agents like iodine and silver trifluoroacetate, enabling stepwise disulfide bridge formation. While synthesising peptide with three disulfide bridges, like growth factors, every pair must bond sequentially to prevent misfolding. Fmoc-Cys(Acm)-Cys(Acm)-OH allows only one bridge to be formed at a time, followed by selective Acm removal to guide the next step.
Orthogonal Protection in multi-disulfide peptides: The Advanced process of Solid Phase Peptide Synthesis requires the orthogonal protection scheme to isolate specific groups for controlled removal of protecting groups. The orthogonal protection offered by Fmoc and Acm groups is especially important for synthesizing peptides requiring two or more disulfide bridges in a defined arrangement. Since Acm is compatible with Fmoc/tBu strategies and stays intact during global deprotection and resin cleavage, it proves to be an ideal group.During the synthesis of cyclic peptides or macrocyclic antibiotics, where one disulfide loop must be formed before the next one, Acm provides temporal control over the reaction pathway.
Designing and developing Peptide-Based Therapeutics: Peptide therapeutics are gaining popularity in treating diseases like cancer, autoimmune disorders, and infectious diseases. These molecules often need cysteine residues for better stability or bioactivity via disulfide formation. The Fmoc-Cys(Acm)-Cys(Acm)-OH dipeptide assures site-specific disulfide formation in bioactive motifs, improves in-vivo stability of linear and cyclic peptides, and supports the design of prodrugs and controlled-release systems.
Synthesis of Cyclic and Loop-Structured Peptides: The disulfide bridges are often used to induce confrontational constraints in peptides so that they can adopt stable loop structures to enhance target specificity. The Fmoc-Cys(Acm)-Cys(Acm)-OH acts as a modular unit for introducing two cysteines in a defined sequence. It also supports the formation of head-to-tail or side-chain-to-side-chain loops. With the use of this dipeptide, you can easily prevent mispairing by keeping thiol groups dormant until needed.
To Construct Peptide Libraries for Drug Screening: Researchers often create combinatorial peptide libraries while researching and developing new lead compounds for drug discovery. The structural integrity of these peptides is the key to obtaining reliable screening results. The Fmoc-Cys(Acm)-Cys(Acm)-OH dipeptide allows the synthesis of libraries containing defined cysteine motifs. It protects the peptide from events like random oxidation, which can distort the structure-activity relationships (SAR). It also supports high-throughput synthesis with predictable folding.
To study Structural Biology and Protein Engineering: Researchers studying enzyme mimetics and protein folding models often require peptides to mimic larger protein domains. These models must have precise disulfide bridge architecture. This dipeptide is a reliable source of protected Cys-Cys dipeptide units that help in modelling domain-specific folding via synthetic approaches. It also supports bioconjugation and PEGylation studies.
Benefits of using Peptide Working Standards
When a highly pure homo-dipeptide is used as a working standard, it renders the following benefits:
Verifying Consistency across Batches: While synthesizing peptides, it is of utmost importance to maintain batch-to-batch consistency. This homodipeptide molecule serves as a control for comparing the retention times in HPLC. Also, it helps to detect any deviation in the synthesis of the protected cysteine sequences while ensuring the purity of the intermediates.
Validation of Analytical Methods: The manufacturers must validate analytical methods such as HPLC, MS, NMR, FTIR, and TGA. When supplied with all the characterization data, the dipeptide makes it a reliable tool for developing methods and validating them in the labs. At Advent, we manufacture this dipeptide on an Mg to G scale with more than 95% purity and provide all characterization data required for validation, such as 1H NMR, Mass, IR, and TGA.
Acts as Control: Before analysing every batch, the systems are tested for performance using peptide working standards. The dipeptide acts as a positive control, confirming that the systems perform within acceptable parameters. When used as a positive control, it helps to eliminate false negatives or positives in the downstream testing.
Training and Developing SOPs: The researchers use peptide working standards like this dipeptide to train analysts on correctly handling and testing protected peptides. It is also used to benchmark chromatographic profiles, purity percentages, and molecular weights.
Where to Buy Fmoc-Cys(Acm)-Cys(Acm)-OH dipeptide?
At Advent, we produce Fmoc-Cys(Acm)-Cys(Acm)-OH dipeptide with more than 95% purity in the Mg to G scale. As mentioned above, we also provide the characterization data for your validation processes.
For more information about this dipeptide, reach out to us at Advent today.