New impurity standard supports method validation, stability testing, and regulatory filings for Lidocaine-based formulations
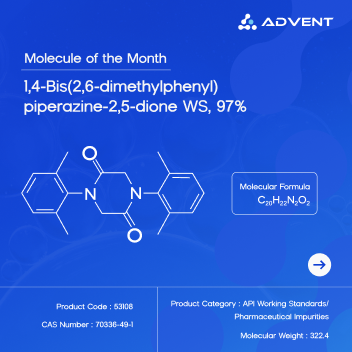
Mumbai, India — Advent, a leading manufacturer of fine and specialty chemicals, announces the latest addition to its pharmaceutical impurity product line: 1,4-Bis(2,6-dimethylphenyl)piperazine-2,5-dione, a key process-related impurity of Lidocaine. This high-purity reference standard reinforces Advent’s commitment to delivering precision, documentation, and regulatory-ready solutions to the pharmaceutical industry.
Lidocaine, a widely used local anesthetic and Class 1b antiarrhythmic agent, is present in various formulations including injectables, patches, gels, and sprays. During its synthesis or degradation, the compound 1,4-Bis(2,6-dimethylphenyl)piperazine-2,5-dione may form as a by-product. Characterized by a stable diketopiperazine ring, this impurity can persist in formulations if not well-controlled. As a result, its routine monitoring is essential to ensure the safety, efficacy, and compliance of Lidocaine-based drug products.
Advent manufactures this impurity standard at a purity level of ≥97%, confirmed using high-performance liquid chromatography (HPLC). Each batch is delivered with a complete analytical package that includes a Certificate of Analysis (CoA), HPLC chromatogram, mass spectrometry (MS) data, proton nuclear magnetic resonance (¹H NMR) spectroscopy, infrared (IR) spectroscopy, and thermogravimetric analysis (TGA) data. The compound is offered in quantities ranging from milligrams to grams, ensuring it is suitable for both laboratory-scale method development and commercial-scale quality control.
This impurity plays a crucial role in addressing regulatory requirements set by international agencies, particularly under the ICH Q3A/B guidelines. It supports a wide range of analytical and regulatory needs. These include chromatographic method development and validation as per ICH Q2 standards, impurity profiling for both APIs and formulations, and conducting forced degradation and long-term stability studies. The impurity also provides a robust reference for regulatory filings such as Drug Master Files (DMFs), Abbreviated New Drug Applications (ANDAs), and New Drug Applications (NDAs). Furthermore, its structural similarity to diketopiperazines linked to enzyme inhibition makes it important in toxicological qualification studies.
“As regulatory expectations become more stringent, our goal is to ensure that our clients are equipped with the tools they need for accurate impurity analysis,” said Amit Singh, the CEO and Co-Founder of Advent. “This Lidocaine impurity standard reflects our ongoing investment in quality, compliance, and scientific integrity.”
About Advent
About Advent is a global supplier of fine and specialty chemicals, with a specialized focus on pharmaceutical impurities, reference standards, and custom synthesis. Known for purity, consistency, and comprehensive documentation, Advent serves pharmaceutical manufacturers, CROs, and research institutions around the world.