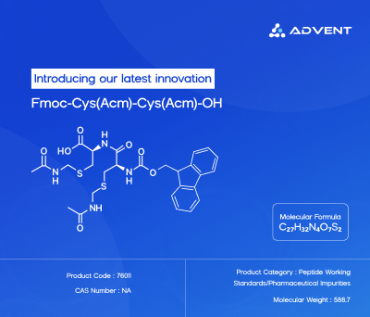
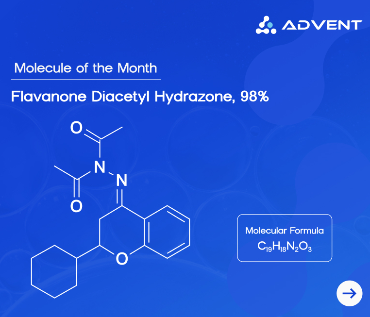
It all starts with a vial. Tiny as it may be, this vial holds the foundation of pharmaceutical quality assurance — a standard. But not all standards are created equal. There are primary, secondary, and reference standards — each playing a vital role in ensuring your drug is safe, stable, and exactly what it says on the label.
Let’s break it down, minus the jargon and plus a little clarity.
Primary Standards
Think of primary standards as the gold standard. Literally. These are highly pure, well-characterized compounds supplied by official bodies like the USP (United States Pharmacopeia), EP (European Pharmacopoeia), or NIST (National Institute of Standards and Technology).
Why are primary standards important? They serve as the baseline — the reference point for everything else. Every calibration, every quantification effort in the lab begins here. They’re expensive, tightly regulated, and available in limited supply.
Applications:
Calibration of analytical instruments
Quantitative and qualitative analysis
Establishing traceability
Secondary Standards
Now, imagine your primary standard had a sibling — just as reliable, but more practical for daily use. That’s your secondary standard. Prepared and qualified against a primary standard, these are more readily available and cost-effective. They're typically manufactured by private laboratories or in-house by pharmaceutical companies.
Why they matter: They’re your go-to for routine analysis. Secondary standards help reduce dependency on costly and limited primary standards, without compromising on accuracy — provided they’re properly validated.
Applications:
Routine quality control testing
Batch-to-batch consistency checks
Daily lab work
For more information on primary and secondary standards, click here.
Reference Standards
Here’s where it gets a bit nuanced. Reference standards can be either primary or secondary — the term “reference” refers more to how they’re used than what they are. They’re used as a benchmark for identity, strength, quality, and purity. So yes, your lab could be using a secondary standard as a reference, as long as it’s appropriately qualified.
Why they matter: They form the backbone of compliance. Whether you're validating a method or testing an impurity, reference standards ensure your data stands up to scrutiny — by regulators and auditors alike.
Applications:
Method validation
Impurity profiling
Regulatory submissions
But Here’s the Catch: Not All Reference Standards Are Created Equal If the reference standard is poorly characterized or unstable, your entire analysis can go sideways. That’s where trusted suppliers come in — and why Advent has become a go-to partner in the diagnostics and pharmaceutical space.
Meet Advent: Your Partner in Purity
At Advent, reference standards aren’t just another product — they’re a promise of precision.
Advent manufactures and distributes high-quality, well-characterized reference materials that meet global standards. Whether you’re testing raw materials, finished products, or developing diagnostic kits, Advent’s reference standards help you maintain compliance and confidence.
Stringently validated against certified primary standards
Available across a wide range of applications — pharma, diagnostics, fine chemicals
Reliable documentation and batch-to-batch consistency
In short, Advent brings you the quality of a primary standard with the practicality of a secondary — so your lab doesn’t miss a beat.
Need help choosing the right standard? Let’s talk — because quality doesn’t happen by accident. It’s measured.