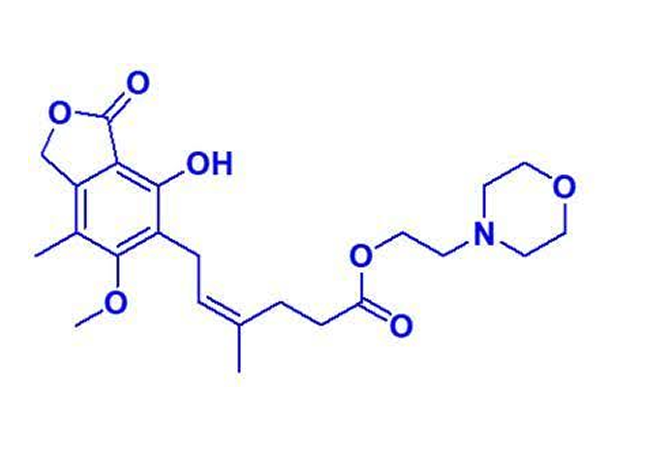
In the complex and highly regulated world of pharmaceuticals, impurities within Active Pharmaceutical Ingredients (APIs) are not merely byproducts to be overlooked—they are critical components that contribute to ensuring the safety, efficacy, and quality of the final pharmaceutical product. Among these, Mycophenolate Mofetil EP Impurity C, chemically known as 2-(Morpholin-4-yl) ethyl (4Z)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate (as per EP), holds a prominent position. Commonly referred to as Z-Mycophenolate Mofetil (USP), this impurity represents a unique intersection of chemical complexity and practical necessity in modern pharmaceutical development and production.
This molecule developed by Team Advent has great benefits in synthesizing crucial impurities. Here’s a look at the molecule.
The Role of Impurities in Pharmaceutical Development
To the uninitiated, the term "impurity" might carry a negative connotation, but in pharmaceutical science, impurities are far from undesirable. Instead, they are meticulously studied, quantified, and leveraged to ensure the robustness of manufacturing processes and the quality of APIs. Regulatory agencies like the USFDA and EMA mandate the characterization, monitoring, and control of impurities, making them indispensable in the pharmaceutical lifecycle.
Mycophenolate Mofetil EP Impurity C, specifically, is the Cis-analogue of the parent API, Mycophenolate Mofetil. Its role extends far beyond being a trace byproduct; it plays a critical role in quality control, analytical method validation, and regulatory compliance. Additionally, its unique chemical structure demands exceptional expertise in synthesis, characterization, and handling, setting it apart from standard impurities.
The Structural Complexity of Impurity C
One of the defining features of Mycophenolate Mofetil EP Impurity C is its structural complexity. Chemically, it is a highly specific compound with distinctive stereochemistry, denoted by the Cis configuration. This structural nuance requires precise synthetic strategies and robust quality assurance protocols to ensure its reliability and reproducibility.
The intricate molecular arrangement of Impurity C, including its morpholin-4-yl group and hydroxy-methoxy-substituted isobenzofuran moiety, challenges even seasoned chemists. Synthesizing such a molecule involves navigating complex reaction mechanisms, optimizing yields, and maintaining high purity levels—all while minimizing byproduct formation. These challenges highlight the technical expertise required to produce Impurity C at a commercially viable scale.
Critical Applications of Mycophenolate Mofetil EP Impurity C
Impurity C is indispensable across multiple stages of pharmaceutical development and production. Its utility spans from research and development to commercial manufacturing, making it a cornerstone in ensuring the quality of Mycophenolate Mofetil API.
Quality Control (QC) Applications
In pharmaceutical quality assurance, Impurity C serves as a critical reference standard for Abbreviated New Drug Applications (ANDAs). Regulatory bodies require the identification and quantification of impurities to approve new drug formulations. By serving as a benchmark for QC, Impurity C enables manufacturers to meet stringent regulatory requirements.
Analytical Method Development
Analytical chemistry forms the backbone of pharmaceutical research, and the development of reliable methods to detect and quantify impurities is essential. Impurity C plays a key role in developing and validating these analytical methods, ensuring their accuracy, sensitivity, and reproducibility.
Commercial Production
During the large-scale manufacturing of Mycophenolate Mofetil, monitoring impurities like Impurity C ensures the consistency and safety of the final product. Manufacturers rely on Impurity C to fine-tune their production processes, optimize yields, and minimize the risk of out-of-specification results.
The Challenges of Synthesizing Impurity C
Producing Mycophenolate Mofetil EP Impurity C is no ordinary task. Its synthesis involves multiple complex steps, each requiring precision and careful control of reaction conditions. The challenge is compounded by its Cis-analogue configuration, which necessitates advanced techniques to maintain stereochemical integrity.
At the critical synthetic step, chemists must employ specialized reagents, catalysts, and reaction pathways to ensure the selective formation of Impurity C. Furthermore, the purification process demands state-of-the-art equipment and techniques to achieve the desired purity level of over 95%. This high-purity standard is essential for its use in quality control and analytical applications, where even trace impurities can compromise results.
From Milligrams to Grams: Scaling Up Synthesis
One of the hallmarks of successful pharmaceutical manufacturing is the ability to scale up production without compromising quality. Mycophenolate Mofetil EP Impurity C has been successfully developed at scales ranging from milligrams to grams, demonstrating the feasibility of large-scale production. This scalability is a testament to the advanced synthetic strategies employed and the meticulous quality control measures in place.
Comprehensive Characterization: A Pillar of Confidence
In the pharmaceutical industry, characterization is as important as synthesis. To ensure the reliability of Impurity C, comprehensive characterization data is provided with each batch. This data serves as a guarantee of its identity, purity, and stability, giving pharmaceutical companies the confidence to integrate it into their processes.
Key characterization techniques include:
1H NMR (Proton Nuclear Magnetic Resonance): Provides detailed insights into the molecular structure and confirms the stereochemistry.
Mass Spectrometry: Confirms the molecular weight and detects any potential impurities.
Infrared (IR) Spectroscopy: Identifies functional groups and verifies chemical bonds.
Thermogravimetric Analysis (TGA): Assesses thermal stability and degradation properties.
These analytical tools ensure that Impurity C meets the highest standards of quality and consistency, making it a reliable choice for pharmaceutical applications.
Serving the Global Pharmaceutical Industry
Mycophenolate Mofetil EP Impurity C is not confined to a single region or market. It is supplied to domestic and international pharmaceutical companies, reflecting its global relevance and demand. As a Working Standard, it supports a wide range of pharmaceutical stakeholders, from innovators to generic manufacturers.
The ability to meet the needs of diverse markets underscores the versatility and importance of Impurity C in the pharmaceutical landscape. By providing high-purity material with robust characterization data, manufacturers can confidently navigate the complexities of regulatory compliance and quality assurance.
Advancing Pharmaceutical Innovation
The significance of Mycophenolate Mofetil EP Impurity C extends beyond its immediate applications. It represents a broader commitment to advancing pharmaceutical innovation and ensuring patient safety. By enabling precise quality control and analytical methods, it contributes to the development of safer, more effective medications.
Moreover, its synthesis and characterization highlight the synergy between chemistry and technology, showcasing how scientific expertise can address the challenges of modern pharmaceutical manufacturing.
Conclusion
In the pharmaceutical world, where precision and quality are non-negotiable, Mycophenolate Mofetil EP Impurity C stands as a vital tool in ensuring the integrity of APIs like Mycophenolate Mofetil. From its critical role in quality control and analytical method development to its contributions to commercial production, this impurity exemplifies the importance of meticulous research, advanced synthesis, and rigorous characterization.
By delivering high-purity Impurity C to global markets, pharmaceutical manufacturers can uphold the highest standards of quality and compliance. In doing so, they not only meet regulatory requirements but also advance the collective goal of improving patient outcomes through safer and more effective medications.
Mycophenolate Mofetil EP Impurity C is more than a chemical compound—it is a cornerstone of pharmaceutical excellence, bridging the gap between scientific innovation and practical application. As the industry continues to evolve, its importance will only grow, reinforcing its position as a key player in the world of pharmaceuticals.