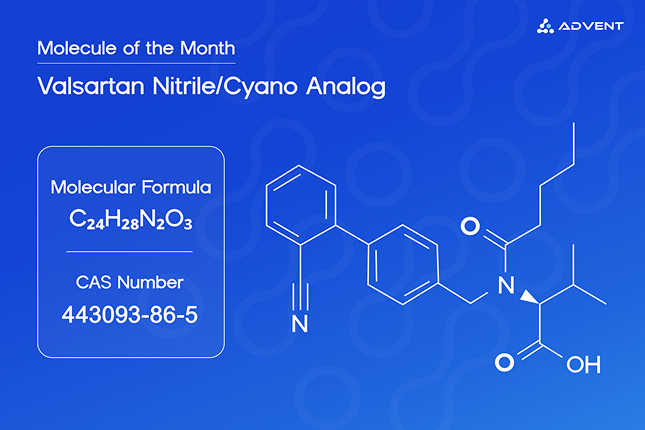
Mumbai, India – [May, 14th 2025] — Advent chembio, a leading developer of pharmaceutical reference standards, has successfully synthesized and scaled the Valsartan Nitrile/Cyano Analog impurity to support global pharmaceutical manufacturers in regulatory compliance and quality control.
Valsartan, a widely prescribed antihypertensive drug, has faced several product recalls in recent years due to the presence of potentially harmful impurities such as nitrosamines and nitrile analogs. Among these, Valsartan Nitrile/Cyano Analog—chemically known as N-Valeryl-N-[(2'-cyanobiphenyl-4-yl)methyl]-L-valine—is a known by-product or degradation impurity formed during the manufacturing process. Though present in trace amounts, it poses potential toxicological concerns and requires careful monitoring as part of regulatory filings such as ANDA (Abbreviated New Drug Application).
To help pharmaceutical companies meet global regulatory expectations, Advent has developed and manufactured this impurity standard with >95% purity at milligram-to-gram scale, making it suitable for method development, validation, and routine quality control activities. The product is available with a complete characterization dossier, including:
¹H NMR (Proton Nuclear Magnetic Resonance)
Mass Spectrometry
Infrared (IR) Spectroscopy
Thermogravimetric Analysis (TGA)
These analytical datasets ensure traceability, structural confirmation, and suitability for use as a working standard in compliance-focused laboratories.
“Impurity profiling is no longer just a quality initiative—it’s a regulatory mandate,” said Dr.Mohanty. “Our Valsartan Nitrile/Cyano Analog standard empowers pharma manufacturers to confidently detect and quantify trace-level impurities, ensuring patient safety and maintaining batch-to-batch consistency.”
The impurity finds critical applications in the development of HPLC and LC-MS methods, determining Limits of Detection (LOD) and Quantitation (LOQ), and forced degradation studies. Its accurate quantification supports product safety, regulatory filings, and market approvals worldwide.
About Advent Fine and Specialty Chemicals
Advent is a trusted manufacturer of high-purity pharmaceutical impurity standards and specialty chemicals. With a global client base and a catalog of over 3,000 reference compounds, Advent supports R&D, quality control, and regulatory compliance needs across the pharmaceutical industry.
For more information or sample requests, visit: Advent Chembio