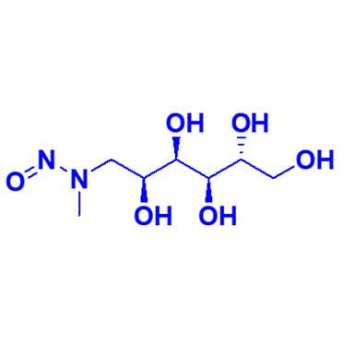
Mumbai, India – June 23, 2025 — Advent, a leading supplier of reference materials and impurity standards, announces the launch of its new high-purity product: N-Nitroso Meglumine. This compound is an important nitrosated impurity associated with the use of meglumine, a widely used pharmaceutical excipient.
The presence of nitrosamine impurities such as N-Nitroso Meglumine has emerged as a significant concern for pharmaceutical manufacturers, following increased regulatory scrutiny by global agencies including the US FDA, EMA, and ICH. These impurities are classified as potentially carcinogenic, making their accurate detection, quantification, and control critical for regulatory compliance and patient safety.
Advent’s N-Nitroso Meglumine is now available for pharmaceutical R&D and quality control use, with a purity level exceeding 95%. The product is supplied with a comprehensive characterization report, including:
1H NMR – Confirmation of proton environment and compound structure
Mass Spectrometry – Molecular weight confirmation
Infrared (IR) Spectroscopy – Functional group identification
Thermogravimetric Analysis (TGA) – Stability and moisture content analysis
This impurity standard supports various applications:
Analysis of meglumine-based formulations during drug development
Quality control for injectable drugs and contrast agents such as iopamidol and iomeprol
Risk assessment and regulatory submissions requiring validated methods for nitrosamine detection
To meet current regulatory expectations under ICH M7 and ICH Q3B, the detection of N-Nitroso Meglumine demands high-sensitivity methods such as HPLC with UV detection, GC-MS, and LC-MS/MS. Advent’s reference standard allows laboratories to develop and validate methods that meet stringent limit of detection (LOD) and limit of quantification (LOQ) requirements—often in the range of ppt to ppb levels.
“Advent continues to expand its portfolio to meet the evolving needs of pharmaceutical quality control and regulatory compliance,” said the CEO and Co-Founder, Amit Singh. “The addition of N-Nitroso Meglumine to our catalogue reinforces our commitment to supporting patient safety and industry standards worldwide.”
This launch adds to Advent’s growing portfolio of over 3000 fine and specialty chemicals, offering global availability and reliable supply for pharma and biotech industries.
Product Information:
Product Name: N-Nitroso Meglumine
CAS Number: 10356-92-0
Molecular Formula: C₇H₁₆N₂O₆
Molecular Weight: 224.21 g/mol
Product Code: 87309
About Advent :
Advent is a trusted supplier of impurity reference standards, specialty chemicals, and analytical reagents. Headquartered in Mumbai, the company serves pharmaceutical and chemical laboratories with high-quality materials for R&D, regulatory submissions, and quality assurance.