Team Advent introduced high-purity N-Nitroso Bisoprolol in March 2025 to serve as an essential impurity reference standard which assists pharmaceutical establishments in maintaining product quality together with compliance and safety requirements.
The high-purity N-Nitroso Bisoprolol’s impurity standard surpasses 95% purity while becoming available as a product for research applications and regulatory use and manufacturing requirements.
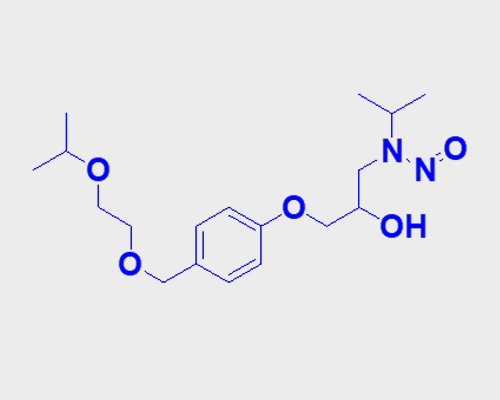
The nitrosated compound, N-Nitroso Bisoprolol exists under the chemical name N-(2-Hydroxy-3-(4-((2-isopropoxyethoxy)methyl)phenoxy)propyl)-N-isopropylnitrous amide when used as an impurity in API Bisoprolol synthetic processes. N-Nitroso Bisoprolol has a Molecular Weight value of 354.44 g/mol alongside the formula C18H30N2O5.
The chemical compound exists as a crucial manufacturing by-product that appears when synthesizing Bisoprolol which serves as a beta-blocker used for hypertension and cardiovascular treatment. Manufacturers need to control N-Nitroso Bisoprolol because it belongs to the N-nitrosamine chemical group and potential carcinogenic effects have raised detection-level concerns throughout production.
The N-Nitroso Bisoprolol chemical product provided by Advent meets the specific quality needs of pharmaceutical manufacturers in their production facilities along with their research operations.
Key Features of Advent’s N-Nitroso Bisoprolol:
High Purity: >95% purity, providing a reliable standard for impurity profiling and detection.
Comprehensive Characterization: Includes detailed spectroscopic data, such as NMR, Mass Spectroscopy, IR, and Thermogravimetric Analysis to confirm the structure, purity, and stability of the chemical.
Support for Compliance: Aids in adherence to global regulatory frameworks, including ICH guidelines and forced degradation studies for risk assessments.
Versatile Applications: Ideal for use in developing analytical methods like LC-MS, GC-MS, and HPLC for detecting and quantifying nitrosamine impurities.
The new chemical product provided by Advent allows pharmaceutical manufacturers to build tested detection systems and maintain formulation safety through research to reduce nitrosamine formation during drug production.
“We are excited to offer the N-Nitroso Bisoprolol to the pharmaceutical community,” said Dr. Rashmi Ranjan Mohanty, Co-founder & Director - Technical at Advent. “With the growing concerns around nitrosamines in the industry, our high-purity N-Nitroso Bisoprolol serves as a crucial tool in ensuring drug safety and regulatory compliance.”
Want to know more about N-Nitroso Bisoprolol? Drop us an email at sales@adventchembio.com or visit www.adventchembio.com.
About Advent: Advent is a Mumbai-based chemicals manufacturing company and a leading supplier of Fine & Specialty chemicals, customized products and performance materials. We cater to the growing developmental and manufacturing needs of various sectors in the Chemical & Pharmaceutical industries.